The therapy centric risk analysis begins with the identification of the hazards and the associated harms for the therapy or procedure. Hazards, a potential source of harm, should be specific to the therapy or procedure. Mapping to the therapy or procedure makes it easier to identify the possible harms associated with the hazard. For example, the hazards associated with a drug delivery therapy can be characterized as
- Over delivery of drug by X% or over
- Over delivery of drug by less than X%
- Under delivery of drug by Y% or over
- Under delivery of drug by less than Y%
- Delay of therapy by over some time
- Delay of therapy by less than some time
- Interruption of therapy by over some time
- Interruption of therapy by less than some time
- Exposure to particulate matter (non-biologic)
- Exposure to biologics
- Exposure to EMI/EMC
- Exposure to electrical current
- Exposure to hot surface
- Physical impact (drop or otherwise)
- Unintended release of patient information
Additional Hazard Identification guidance can be found in EIN 60601-1 3rd edition in the risk management tables. This list should be modified or extended for the specific medical application, but many of these apply to all devices.
As noted, linking the hazards to the therapy or procedure allows a simpler mapping of harms to each hazard. Harms represent the impact of a hazard, and with the understanding of the therapy, the understanding of the harm is simplified. Each harm has an impact on a patient or clinician, and this impact has a severity. Severity for harms is usually characterized as follows
Severity of Harm
| Severity of Harm | Description |
|---|---|
| Catastrophic | Results in patient death. |
| Critical | Results in permanent impairment or life-threatening injury. |
| Serious | Results in injury or impairment requiring professional medical intervention. |
| Minor | Results in temporary injury or impairment not requiring professional medical intervention. |
| Negligible | Little or no injury |
Each specific patient or user harm may have a wide range of impacts, with each impact having a specific severity and probability. As an example, infections due to biologics as a result of a therapy may have little impact (negligible) or may lead to death (catastrophic) in some rare cases. The distribution of impact is a probability distribution as shown in the following table.
Harm Severity
| Harm | Severity | ||||
|---|---|---|---|---|---|
| Catastrophic | Critical | Serious | Minor | Negligible | |
| Infection | 0.000010 | 0.00004 | 0.00005 | 0.40000 | 0.59990 |
The harm severity distribution should be developed based upon historical clinical data and a detailed analysis of the therapy or procedure.
After the identification of clinical hazards and the characterization of harms, harms are mapped to the hazards. The mapping must consider that a single hazard may result multiple harms. As an example, over delivery may result in several harms, with each harm will having an associated distribution of severity. The following table shows the linking of harms severity distributions to a specific hazard.
| Harm Likelihood | Severity of Harm Probability | |||||
|---|---|---|---|---|---|---|
| Hazard – Over Delivery | Catastrophic | Critical | Serious | Minor | Negligible | |
| Associated Harm #1 | 0.5 | 0.000010 | 0.00004 | 0.00005 | 0.40000 | 0.59990 |
| Associated Harm #2 | 0.5 | 0.000010 | 0.00004 | 0.00005 | 0.30000 | 0.69990 |
The weight is the likelihood of the particular harm resulting from the specific hazard. This weight allows the development of an aggregated probability for each severity level. The aggregated probability of each severity can be characterized as shown below.
Hazard Severity Probability Distribution
| Severity | |||||
|---|---|---|---|---|---|
| Hazard | Catastrophic | Critical | Serious | Minor | Negligilbe |
| Over Delivery | 0.000010 | 0.00004 | 0.00005 | 0.35 | 0.6499 |
In this case the sum of probabilities across all of the severities is one, that is, the distribution of probability for a given hazard against the severities must sum to 1.
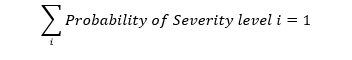
An alternative qualitative approach assigns a single, most likely severity to each hazard. With this qualitative approach, care must be taken assigning severity. The tendency during severity analysis is to assign a severity of “catastrophic” because of some small but finite probability of death exists. This tendency for a qualitative approach to default to a “catastrophic/improbable” severity/probability combination for a given hazard will distort subsequent hazard analysis. Even with a qualitative approach, awareness that each hazard results in a variety of harms and severities is needed to assign the “most likely” severity
The medical team most often performs this activity of identifying hazards and mapping harms, as the linkage and understanding of harms relates to the therapy or procedure, that is, the medical uses. Probabilities of severity should be established with extensive clinical research.
At the completion of this step the Hazard Identification Table, as shown below, can be completed.
| Hazard Identification Element | Description |
|---|---|
| Hazard | Short description of Hazard. |
| Associated Harm(s) | Harm which may occur if Hazard is present. |
| Probability of Harm (P2) | The probability of occurrence of harm given the hazardous situation is present. This may either the most likely value or a distribution as shown in the Hazard Severity Probability Distribution |
| Severity of Harm | Severity classification of the harm. This may either be a most likely value or a distribution as shown in Hazard Severity Probability Distribution |
 TGFR Consulting LLC
TGFR Consulting LLC