Elaborating the concept integrates the results of the concept definition into a complete system definition, the design inputs.
Concept Elaboration SIPOC
| Inputs | Key Activities | Outputs |
| Clinical Use Clear understanding of the overall therapy, workflow and risks associated with the seminal idea Selected Concept The concept output, including the CTQs from the concept selection process Standards Regulatory as well as business standards consistent with the markets for the product Manufacturing Capability Characterization of capacity, measurement systems and process capability | Task and Preliminary Hazard Analysis a structured analysis of the clinical application to develop system modes of operations and the hazards associated with the use of the system. Architecture Elaboration Breaking the system down to into subsystems Requirements Elaboration Translation of the Performance Parameters, CTQs and concept into the system definition (use cases, requirements, architecture and interfaces) Final Concept Confirmation The final confirmation of the concept with the users | System Definition (Design Inputs) · Task Analysis · Preliminary Hazard Analysis · System Architecture · Use Specifications · System Requirements · Interface Requirements |
The overall workflow is as shown in the following
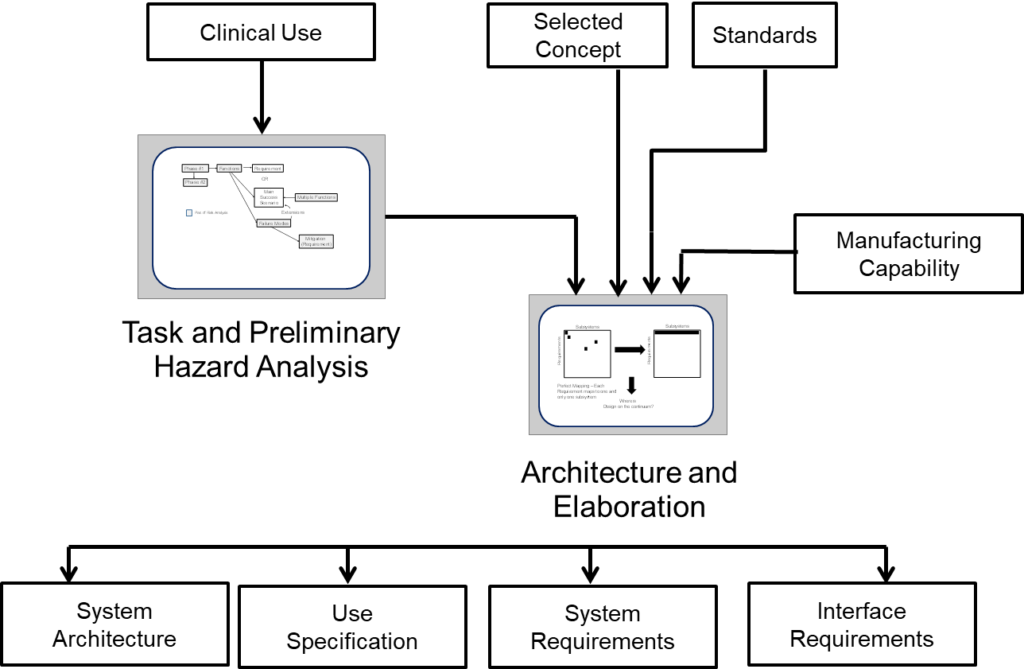
Architecture and Elaboration translates the inputs into requirements, integrating the selected concept with regulatory standards, the VOB and Manufacturing Capability as well as the tasks and risk controls defined by the intended use. The finished requirements define the device and will serve as the basis for all other design and development activities.
 TGFR Consulting LLC
TGFR Consulting LLC