Task analysis breaks down the use of the device into discrete sequences of functions or tasks. This analysis considers the complete therapy to gain a more complete view of the therapy delivery as well as interaction of the user, patient, and device. This complete view of the therapy drives all subsequent risk and design activities.
Identifying the Mission Phases
The first process step identifies the overall “mission”. In most cases the “mission” is the delivery of a single therapy or execution of a single procedure.
The following figure presents a pictorial sequence of a single “mission”, consisting of the operations associated with a single drug delivery.
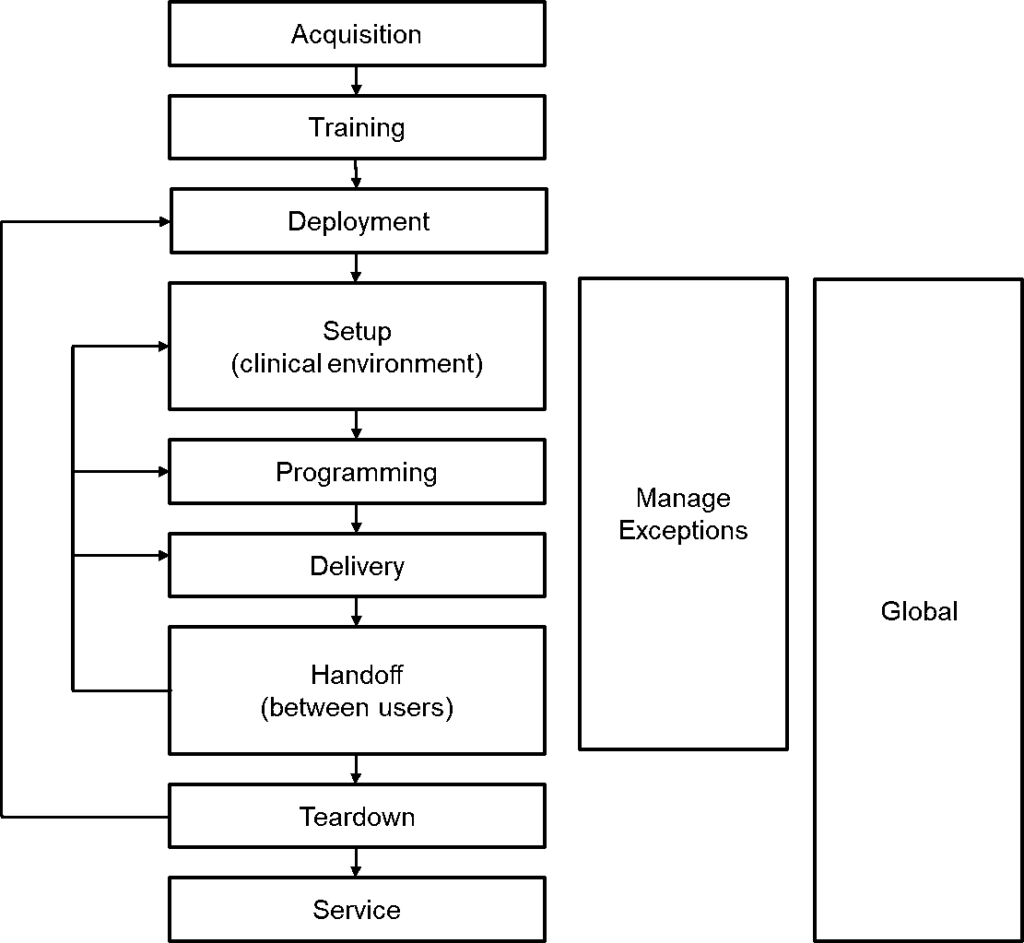
These phases associated with drug delivery can easily be modified and applied to all manner of devices. The following table summarizes each operational phase identified and the possible failure modes
| Phase | Description |
| Acquisition | The device ships from the manufacturer to the user. Possible failure modes would include shipping damage |
| Training | The training of the users Possible failure modes would include to complete training |
| Deployment | Moving the device to a position of use, such as loading a disposable into the COW (computer on wheels) Possible failure modes might be storing a prefilled device in the wrong dose bin due to labeling or physical damage to the device while pushing a device on wheels. |
| Setup | Setting up for delivery of the procedure. Possible failure modes might include improper configuration by the clinician |
| Programming | Programming the device for the procedure. Possible failure modes might include setting the wrong dose |
| Delivery | Delivering the therapy. This may be modified depending upon the therapy Possible failure modes might include failure of the device to deliver |
| Handoff between users | This is the presentation of status during shift changes. Possible failure modes might be failure of the clinicians to discuss status when changing shifts. |
| Teardown | This is tearing down the setup for the procedure. Possible failure modes might include sharp objects being exposed |
| Service | This includes cleaning and service. Possible failure modes might include allowing cleaning solutions into the device |
| Manage Exceptions | Handling alarms and issues. Exceptions are generally handled outside of normal operational context. Possible failure modes might include unnecessary interruption of the therapy |
| Global | Global includes things such delivering electrical power – those things that move across other phases Possible failure modes might include battery depletion |
Identifying Actors
When defining the operational phases, consider phases based upon the actors performing the functions or tasks. In the expanded task analysis, the actors include
| Actor | Description and Actions |
| Administrator | The administrator usually handles the Acquisition, Training and Deployment |
| Clinician | The clinician handles the setup, programming, handoff between users, managing exceptions, and teardown |
| Device | The device handles delivery of the therapy and many of the global tasks |
| Bio-Med Technician | The bio-med technician handles service, including the cleaning. |
Functions
Once operational phases have been identified, the next step details the functions for each operational phase. Each phase should be decomposed to 10-12 constituent functions, as shown below

The functions may be tasks performed by the user or functions performed by the device, which is why the more general term of “function” is applied.
Failure Modes
Following function identification, a multi-disciplined team identifies the failure modes for each task or function. Failures are initiating events that will eventually lead to a hazard and trigger a hazardous situation. Initially, identify all failure modes, including device and user errors for every function in the “mission” (the delivery of the therapy or treatment). Next steps associate hazards, sequence of events and hazardous situations to these failure modes. At present task analysis focuses on the failure modes for the “mission” functions and tasks
Further Reading
| Topic | Reference |
| Human Factors and Task Analysis | IEC. (2015). IEC 62366-1:2015 Medical devices — Part 1: Application of usability engineering to medical devices. ISO. |
| Human Factors and Task Analysis | Applying Human Factors and Usability Engineering to Medical Devices Guidance for Industry and Food and Drug Administration Staff FEBRUARY 2016 https://www.fda.gov/regulatory-information/search-fda-guidance-documents/applying-human-factors-and-usability-engineering-medical-devices |
| Mission Phases | Barba, R. J. (2004). Failure Mode Effect Analysis Applied to the Use of Infusion Pumps. Proceedings of the 26th Annual International Conference of the IEEE EMBS. |
 TGFR Consulting LLC
TGFR Consulting LLC