The 2nd edition of the Cascade, Building a Medical Device, details a process of developing a medical device, taking the reader from the initial idea through to the realization of the complete medical device. Along the way, the reader will be introduced to a wide range of tools and techniques that will facilitate each of the critical activities. While these tools do not represent the only tools that can be employed, these tools are simple and have been shown to work. With this process outline and proven tools in this blog, the development of a medical device becomes simple, straightforward, and deterministic.
Several things have changed since the 1st edition of the blog. In most cases regulations have aligned with best practices to make the process clearer. In other areas, experience has led to revised tools that make the process simpler. This edition reflects those changes.
The process described here breaks down into two distinct cycles based upon an old paradigm:
- Building the Right Device – defining the scope of the project and establishing the right requirements for the device
- Building the Device Right – executing on the development to create a robust design that meets the requirements for the device.
Building the Device Right
The process of building the right device starts with the Voice of the Customer (VOC) and the Voice of the Business (VOB) and defines the characteristics of a device must meet to satisfy to meet the customer and the business. Concept elaboration creates device concept that meets these characteristics as well as the needs of the medical intended use, regulatory standards. Every concept considers current manufacturing capability, whether internal or external. Finally, the concept is transformed into the system level requirements that define the device at a detail that allows the device to be built right. This is illustrated in the following figure
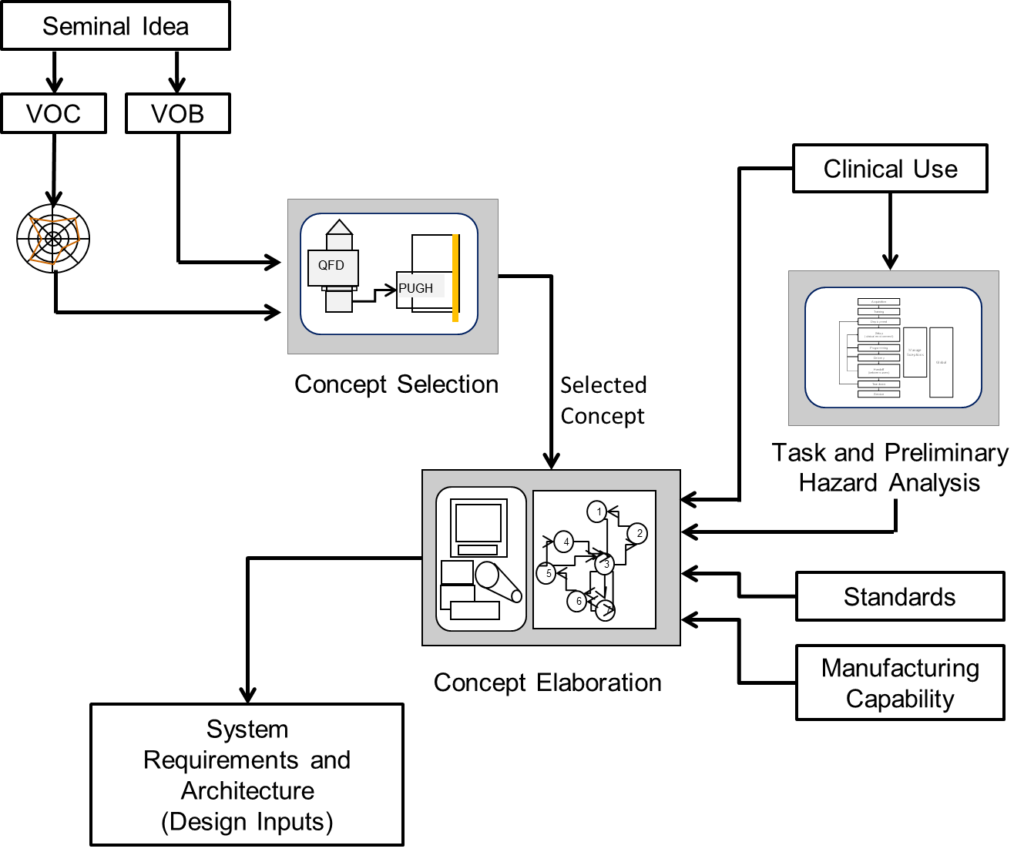
The following table details the tools used to realize the system requirements and use
| Tool | Use |
| Five Whys and User Needs Statements | Translate the Voice of the Customer into actionable user needs |
| The Spider Chart | Map the current condition, the best in class and device goals for the development against the user needs |
| The QFD | Translate user needs into CTQs, device features that determine the success of the product |
| Risk Analysis | Establishing the risk controls that will ensure a safe and effective product |
| The PUGH Matrix | Drive the objective concept selection |
Building the Device Right
Building the Device Right takes the concept, architecture and system requirements and translates these documents into a robust design, free of defects. Requirements translate to drawing to components, to assemblies and into the finished device. Verification confirms that the design meets the requirements, and validation confirms that the finished device meet the stated user needs and intended use.
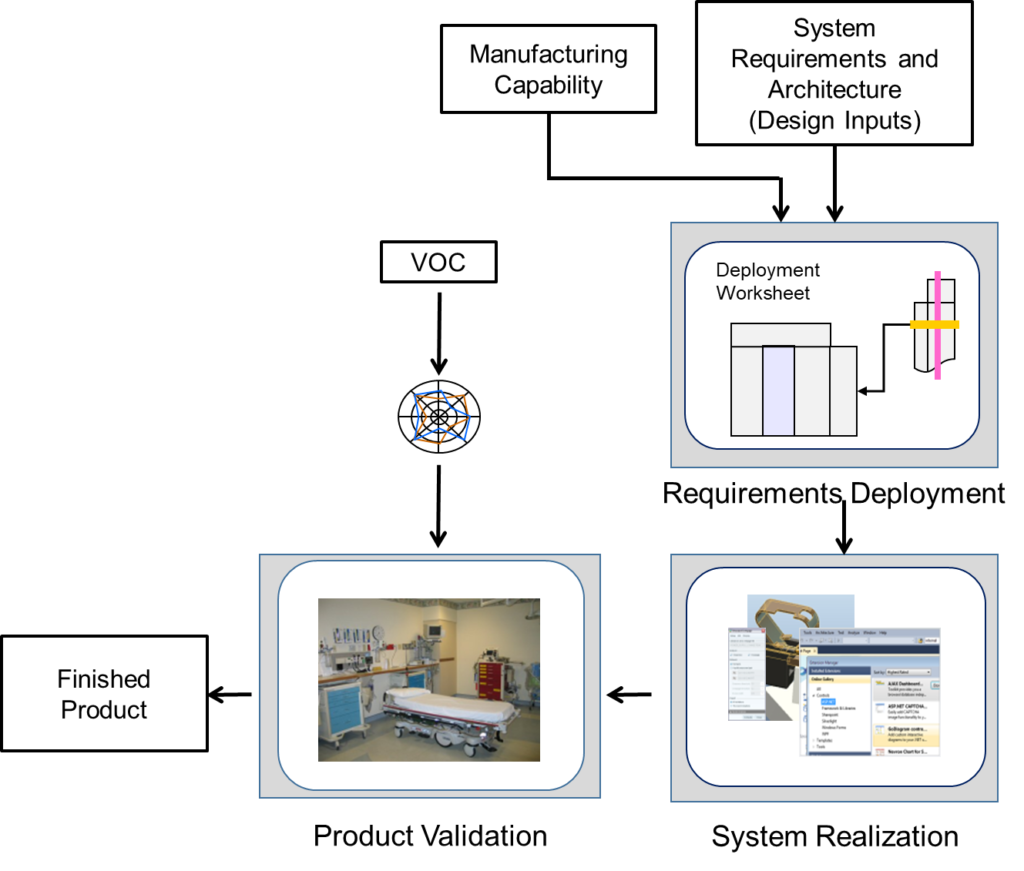
The following table details the tools used to realize the final device
| Tool | Use |
| Requirements Flow down with Transfer Function | Using the concept of a transfer function to map system to subsystem requirements |
| Deployment Worksheet | Ensuring the consistency of the requirements flow down |
| N2 Chart | Identifying the interactions between components of subsystems |
| Use Cases | Defining the user interaction with the design |
| Human Factors Testing | Ensuring the system meets the user needs |
Subsequent blogs will explore each of these activities, highlighting the tools and processes that will ensure a positive outcome. Please join us for the journey
 TGFR Consulting LLC
TGFR Consulting LLC