Over the next several months, this blog will explore the process of developing a medical device. We will explore in detail the steps that transform a great idea into a great product, quickly and compliantly. The process of developing a great medical device can be subdivided into two high level activities
- Creating the Requirements – translating the Voice of the Customer (VOC) and the Voice of the Business (VOB) into meaningful requirements (Design Inputs)
- Developing the Product – translating the requirements into a finished device
There are a number of steps within each of these activities, everything from risk management to system validation. We will explore these steps and attempt to clearly identify how these steps connect and interact, creating a optimum process for the overall development. Key to the process is the concept that work in one step feeds forward to later steps, simplifying the future work. This feed forward mechanism ensures maximum efficiency in the overall process, because artifacts at each step are only developed to be used in future steps. Documentation for documentation’s sake does not exist.
The process of creating the requirements for the project starts with the Voice of the Customer (VOC) and the Voice of the Business (VOB) as shown in the following figure.
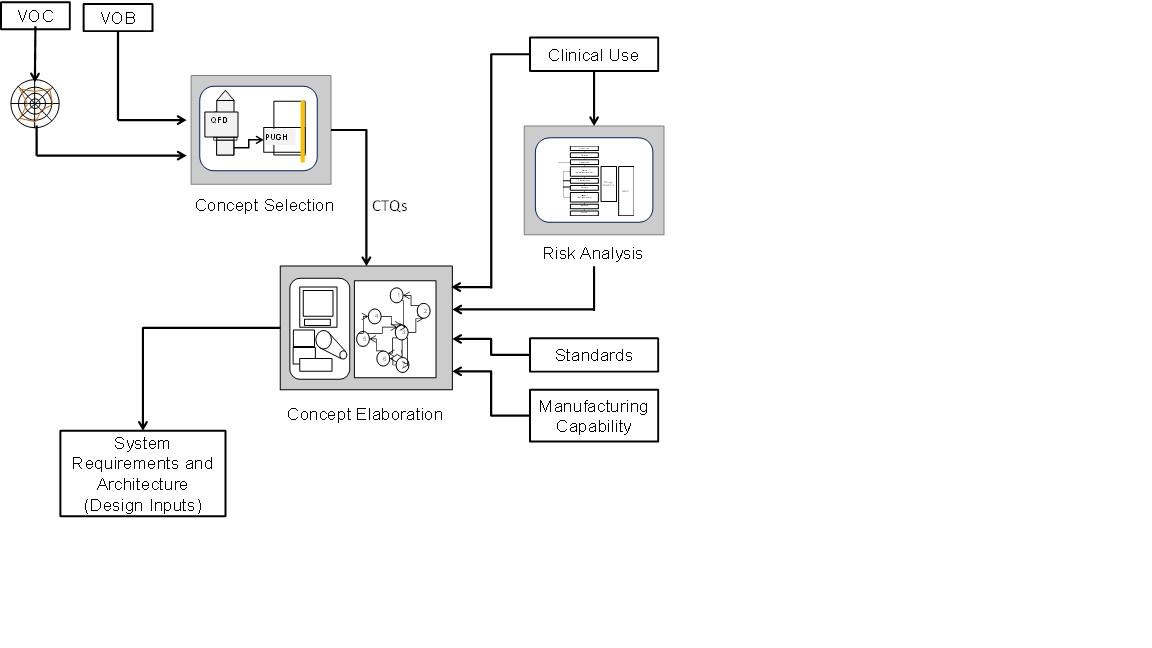
The requirements starts with the requirements process uses the following key tools to drive toward the a product concept
- The QFD (Quality Function Deployment) to develop the Critical to Quality (CTQ) Features for the concept
- The Pugh Matrix and Super Concept to determine the final Concept
Based upon the final concept, the next step in the process elaborates the concept into a final set of requirements that characterize the product and guide all further development. At this stage in the workflow, the elaboration process focuses on the development of the full range of system level the requirements incorporating all of the various sources (CTQs, Regulatory Standards, Risk and Manufacturing Capability).
The final step of the requirements elaboration step confirms the final concept with the customers, users and the business. With the confirmation of the concept, architecture and associated requirements, the process can move on to development.
Developing the product transforms the concept, architecture and system requirements into the medical device, which after validation represents a finished product. The following figure illustrates the development workflow
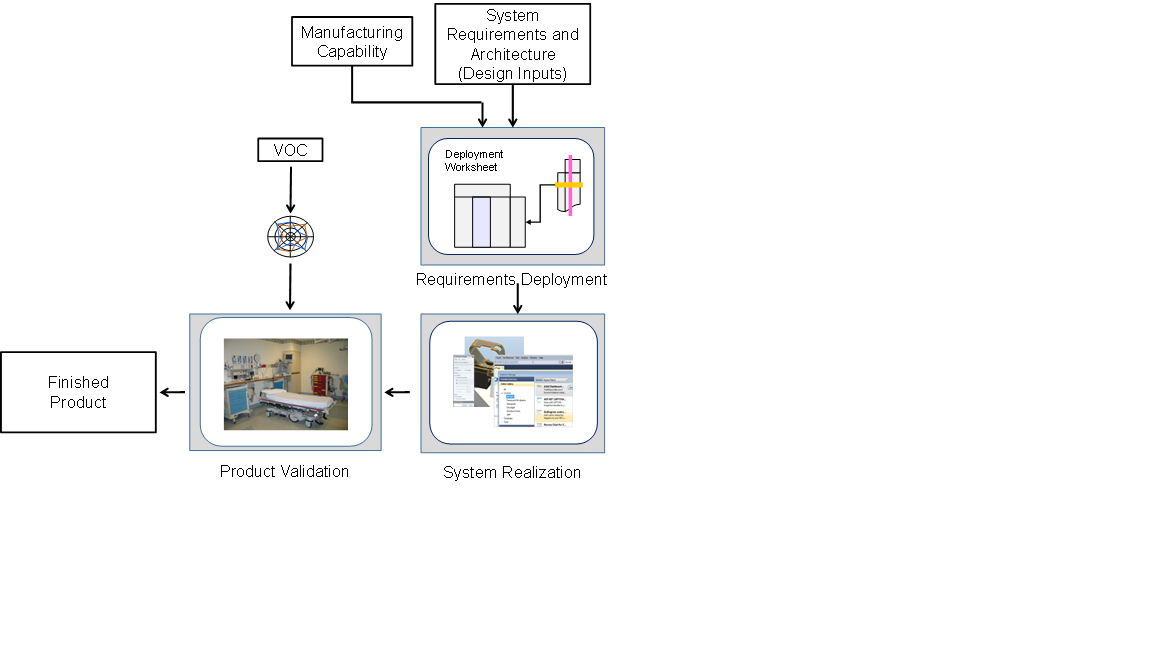
Figure 2 – Developing the Product
The initiating step in development, Deployment, uses the architecture to partition and flow down the system requirements to the subsystems. Deployment worksheets connect the elements of the system and provide the framework for the system realization. System realization consists of detailed subsystem design and development, system integration and the final formal verification of the design. Upon completion of realization, design validation confirms that the device meets the original Voice of the Customer (VOC) and user needs, as set forth during the requirements development activities.
As stated, in future posts we will dive into these activities and show how the unique feed forward of work drives efficiency and completeness. Stay tuned
 TGFR Consulting LLC
TGFR Consulting LLC