Task and preliminary hazard analysis identify the risk controls that a concept must implement to provide adequate product safety.
| Inputs | Key Activities | Outputs |
| Clinical Use The clinical use of the device Clinical Use Error Data Data on the use errors and rates associated with the data. Clinical Hazards and Harms The clinical hazards and harms associated with the therapy. | Phases and Function Identification Describing of the phases and the associated functions associated with the delivery of a single therapy or exam. Task Analysis Identifying the tasks and failure modes associated with the functions. Hazardous Situation Development Identifying the hazards, sequence of events and the hazardous situations Harm Identification Linking the failure harms to hazardous situations and assigning probabilities and severities. Mitigation Development The development of the essential requirements, the requirements that mitigate risks. | Risk Controls The mitigations associated with the inherent risks of the therapy. Task Analysis The tasks and failure modes associated with the functions. |
Task Analysis
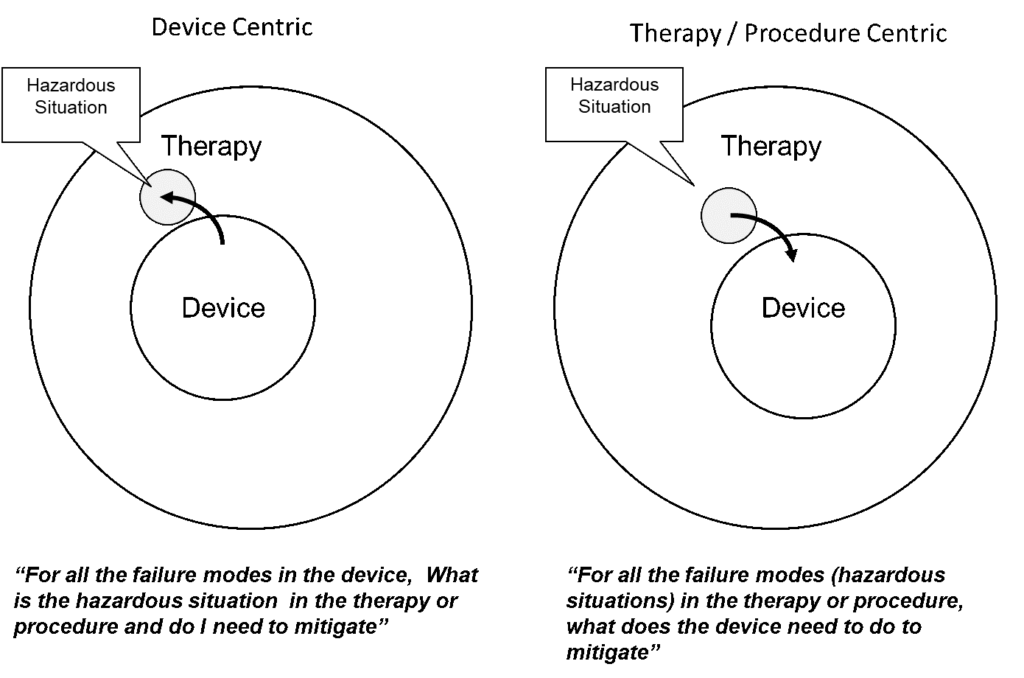
Task Analysis, as described in IEC 62366, Medical devices – Part 1: Application of usability engineering to medical devices, recommends the use of task analysis to identify use errors that could occur and are related to user interface. Extending task analysis to the complete therapy cycle (e.g. shipping to disposal) and adding device failures, makes this a much more effective tool. In addition, a therapy or procedure-based approach that considers all hazardous situations makes the analysis much more complete. This therapy centric approach differs from most previous approaches, which took a device centric approach relative to the identification of use errors, hazardous situations, and harms. As shown in the following figure, the device centric approach does not address the full range of possible hazardous situations associated with the therapy or procedure
Criticality Analysis
Criticality analysis extends the therapy centric risk process by integrating with the task analysis. In keeping with its origins relative to risk and military actions, criticality analysis focuses on steps needed to execute a “mission” and the failures that can impact the execution. With criticality analysis, a failure links to the impact upon the overall “mission”. The same failure can have different criticality based upon what mission activity or operational phase is associated with the failure. This establishes the following linkage.
Failure -> Mission Activity -> Mission Impact
In a therapy centric approach to risk analysis, the “mission” is the delivery of a single therapy or execution of a single procedure. Mission impact is the harm to which the patient may be exposed. In the context of ISO 14971 definitions, criticality analysis systematically maps failures to hazards, hazardous situations, and the overall therapy. The basic flow follows that of Annex E of ISO 14971, shown below
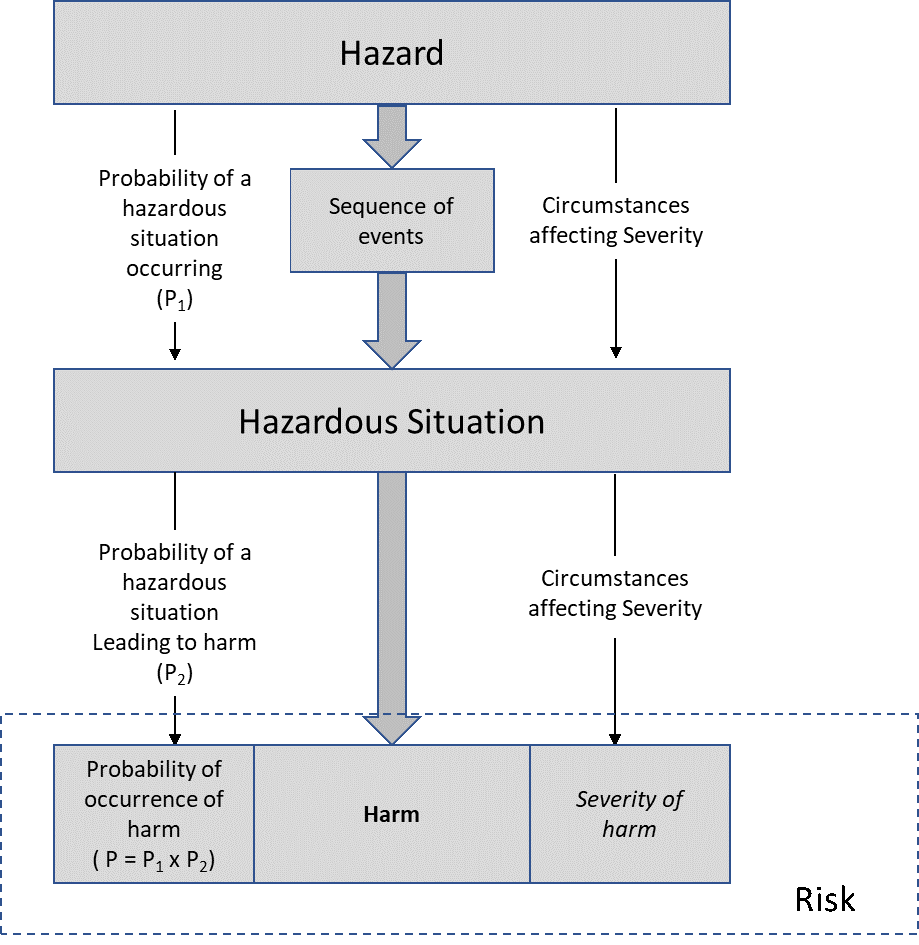
Specifically, application proceeds through the following steps
- Identify all the failure modes associated with the operational phases and tasks and
- Map failure modes to a hazard, a potential source of harm
- Describe the sequence of events leading to a hazardous situation, a circumstance in which people property or the environment are exposed to one or more hazard(s).
- Determine the criticality (harm) for the hazardous situation (as determined by the operational phase and task)
- Identify mitigations when necessary
Criticality Analysis is a natural mechanism for performing preliminary hazard analysis, integrating task analysis with the identification of the hazardous situations and mitigations.
Further Reading
| Topic | Reference |
| Human Factors and Task Analysis | IEC. (2015). IEC 62366-1:2015 Medical devices — Part 1: Application of usability engineering to medical devices ISO. |
| Human Factors and Task Analysis | Applying Human Factors and Usability Engineering to Medical Devices Guidance for Industry and Food and Drug Administration Staff FEBRUARY 2016 https://www.fda.gov/regulatory-information/search-fda-guidance-documents/applying-human-factors-and-usability-engineering-medical-devices |
| Criticality Analysis | Mil-Std-1629A Procedure for performing a failure mode, effects and criticality analysis https://www.fmea-fmeca.com/milstd1629.pdf |
 TGFR Consulting LLC
TGFR Consulting LLC