Medical Device development demands good system engineering and understanding the regulations, risk and clinical use that are unique to the medical device market. To get it right, the focus needs to be on a process that minimizes mistakes and maximizes success.
The medical device development process breaks down into two distinct cycles
- Product Definition – defining the scope of the project and establishing the right requirements for the device
- Design and Development – executing on the development to create a robust design that meets the requirements for the device.
Product Definition – Building the Right Device
The product definition process ensures that the product definition meets the needs of the business and customer. With medical devices, the right product can have a long and profitable life, whereas a product that does not meet these needs is a very wasteful proposition.
The process starts with a product idea, and this product idea guides collecting information that will eventually define the product. Voice of the Customer (VOC) and the Voice of the Business (VOB) define user and business needs necessary to create a successful product. From these needs, the process identifies the key features that will make the product idea a success in the marketplace.
Concept elaboration translates the key features, medical use, regulatory standards, and manufacturing capability into a full product concept. Once the concept is defined, elaboration ensures that this concept meet the needs of the customer and business.
As an output of elaboration, the system level requirements and architecture represent the translation of the concept into the details needed to create the finished medical device.
The following figure outlines the product definition workflow
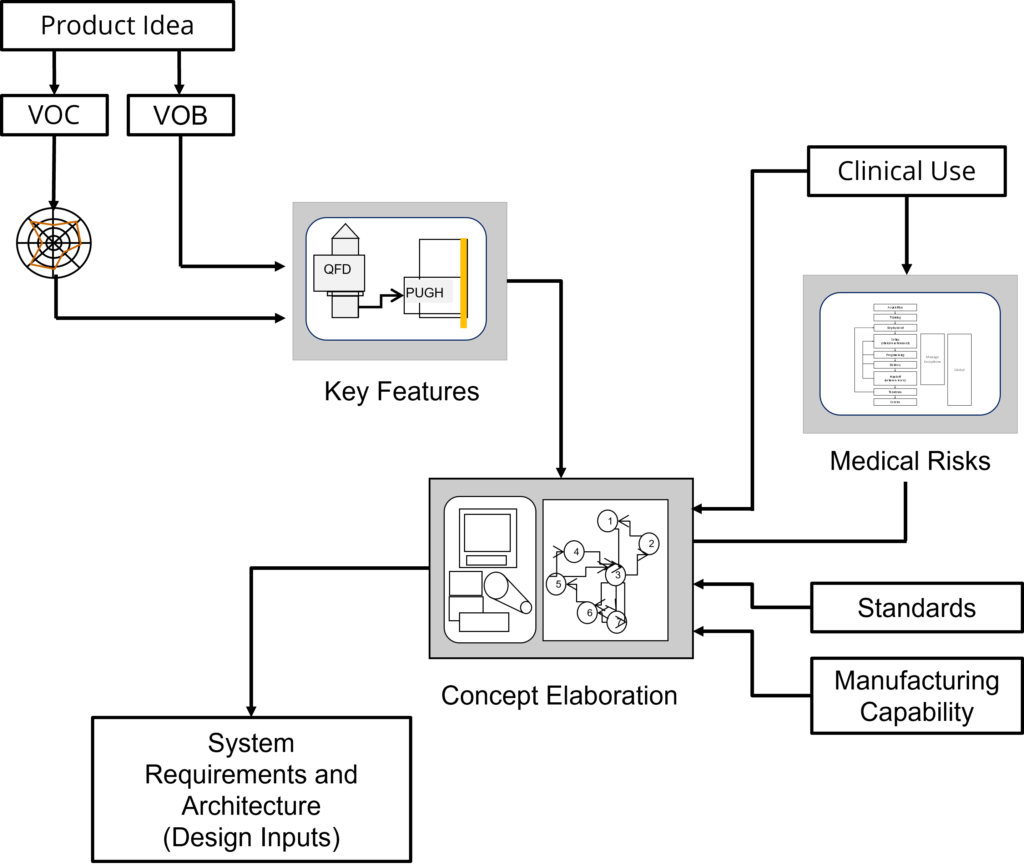
Key Product Definition topics include.
- Developing VOC (Voice of the Customer) and VOB (Voice of the Business)
- Identifying the Key features that will make the product a winner in the marketplace
- Characterizing medical use and risk
- Elaborating the concept and retiring development risks
- Confirming the concept meets the VOC
- Translating a concept into the right requirements.
Design and Development – Building the Device Right
Design and development cascades the product definition down into requirements and specifications for lower-level subsystems and components, designs and verifies the product, and finally confirms that the product meets the user needs.
System Realization cascades the system requirements down through the subsystems and components, all the while preserving linkage up to the parent requirements. Requirements and specifications are then translated into a robust design, free of defects.
While developing the design, integration activities ensure that the subsystems and components work together. All through the design and integration manufacturing develops the process to fabricate and assemble the finished product.
Following realization, verification confirms the product was built right, and validation confirms that the device built still meets the user and clinical needs in a safe and effective manner. This is illustrated in the following figure
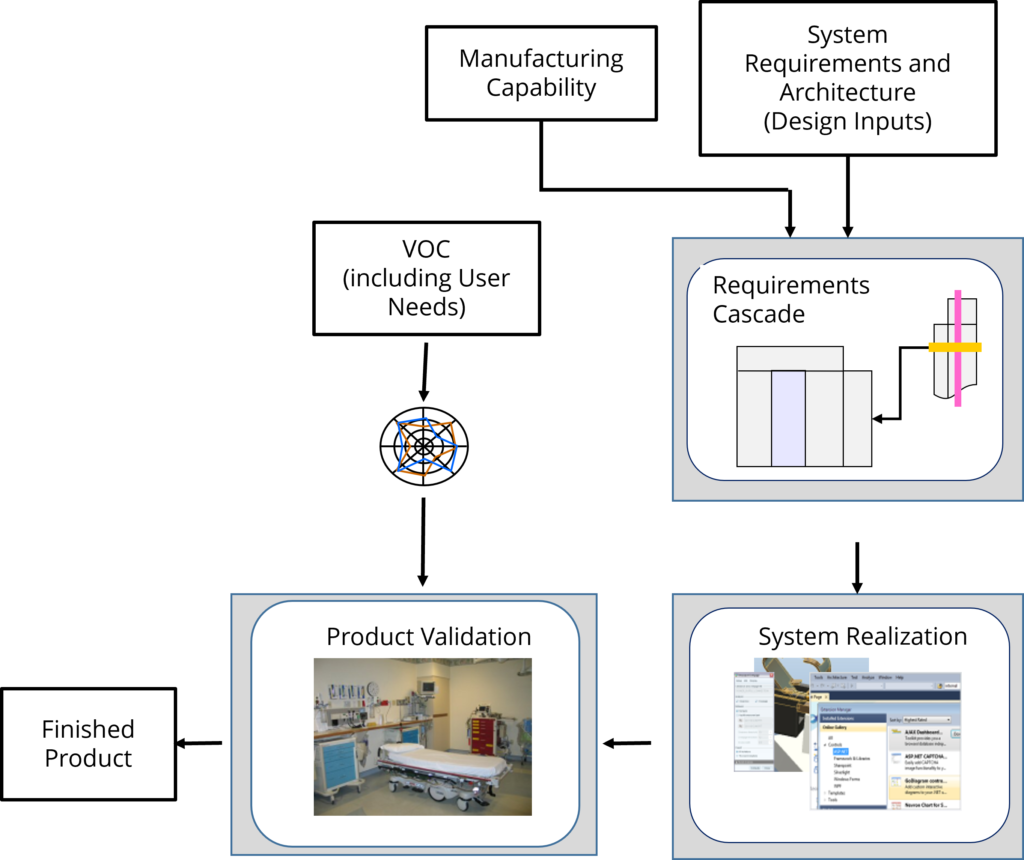
Key “device right” topics include.
- The requirements cascade from top-level to component
- System realization including robust design, integration and design transfer
- System verification
- Product validation, including user interface testing
- Preparing for product launch
Upcoming blogs will explore key parts of the processes supporting product definition and product development, that is, “building the right device” and “building the device right”. Each blog highlights tools and processes needed to ensure that the product is a success in the marketplace and delivers the highest quality. Please join us for the journey
 TGFR Consulting LLC
TGFR Consulting LLC